Don't miss our upcoming virtual workshop with John Snow Labs, Improve Drug Safety with NLP, to learn more about our joint NLP solution accelerator for adverse drug event detection.
The World Health Organization defines pharmacovigilance as "the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine/vaccine-related problem." In other words, drug safety.
Pharmacovigilance: drug safety monitoring in the real-world
While all medicines and vaccines undergo rigorous testing for safety and efficacy in clinical trials, certain side effects may only emerge once these products are used by a larger and more diverse patient population, including people with other concurrent diseases.
To support ongoing drug safety, biopharmaceutical manufacturers must report adverse drug events (ADEs) to regulatory agencies, such as the US Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in the EU. Adverse drug reactions or events are medical problems that occur during treatment with a drug or therapy. Of note, ADEs do not necessarily have a casual relationship with the treatment. But in aggregate, the proactive reporting of adverse events is a key part of the signal detection system used to ensure drug safety.
Adverse event detection requires the right data foundation
Monitoring patient safety is becoming more complex as more data is collected. In fact, less than 5% of ADEs are reported via official channels and the vast majority are captured in free-text channels: emails and phone calls to patient support centers, social media posts, sales conversations between clinicians and pharma sales reps, online patient forums, and so on.
Robust drug safety monitoring requires manufacturers, pharmaceutical companies and drug safety groups to monitor and analyze unstructured medical text from a variety of jargons, formats, channels and languages. To do this effectively, organizations need a modern, scalable data and AI platform that can provide scientifically rigorous, near real-time insights.
The path forward begins with the Databricks Lakehouse, a modern data platform that combines the best elements of a data warehouse with the low-cost, flexibility and scale of a cloud data lake. This new, simplified architecture enables healthcare providers and life sciences organizations to bring together all their data—structured (like diagnoses and procedure codes found in EMRs), semi-structured (like clinical notes) and unstructured (like images)— into a single, high-performance platform for both traditional analytics and data science.
Building on these capabilities, Databricks has partnered with John Snow Labs, the leader in healthcare natural language process (NLP), to provide a robust set of NLP tools tailored for healthcare text. This is critical, as much of the data used for adverse event detection is text-based. You can learn more about our partnership with John Snow in our previous blog, Applying Natural Language Processing to Health Text at Scale.
Solution accelerator for adverse drug event detection
To help organizations monitor drug safety issues, Databricks and John Snow Labs built a solution accelerator notebook for ADE using NLP. As demonstrated in our previous blog, by leveraging the Databricks Lakehouse Platform, we can use pre-trained NLP models to extract highly-specialized structures from unstructured text and build powerful analytics and dashboards for different personas. In this solution accelerator, we show how to use pre-trained models to process conversational text, extract adverse events and drug information and build a Lakehouse for pharmacovigilance that powers various downstream use cases.
The solution accelerator follows 4 basic steps:
- Ingest unstructured medical text at scale.
- Use pre-trained NLP models to extract useful information such as adverse events (e.g., renal damage), drug names and timing of the events in near real-time.
- Correlate adverse events with drug entities to establish a relationship.
- Measure frequency of events to determine significance.
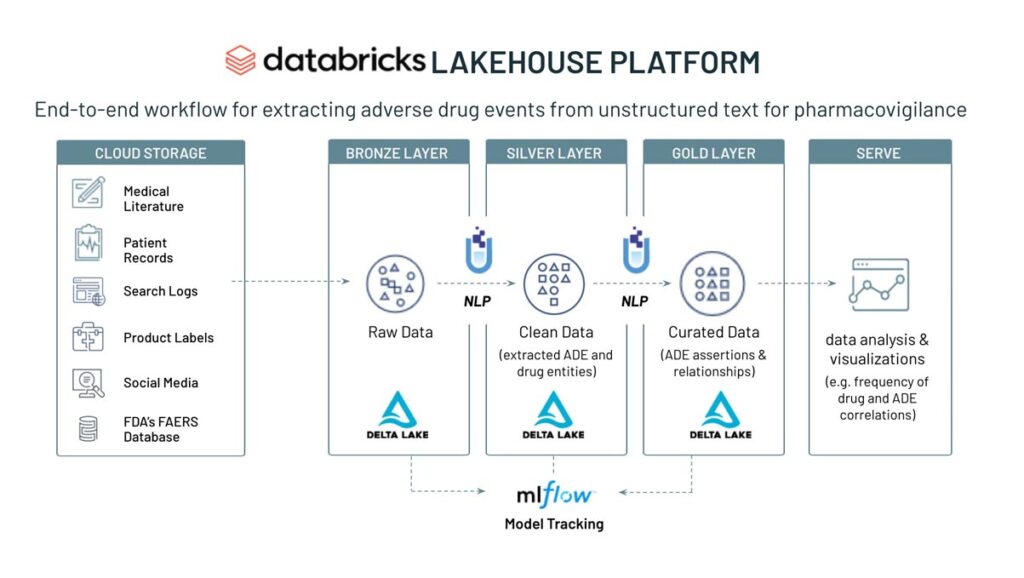
Below is a brief summary of the workflow contained within the notebook.
Overview of the adverse drug event detection workflow
Starting with raw text data, we use a corpus of 20,000 texts with known ADE status (4,200 texts containing ADE) and apply a pre-trained biobert model to detect ADE status and assess the specificity and sensitivity of the model based on the ground truth and the confidence level in accuracy of the assignment. In addition, we extract ADE status and drug entities from the conversational texts by using a combination of ner_ade_clinical and ner_posology models.
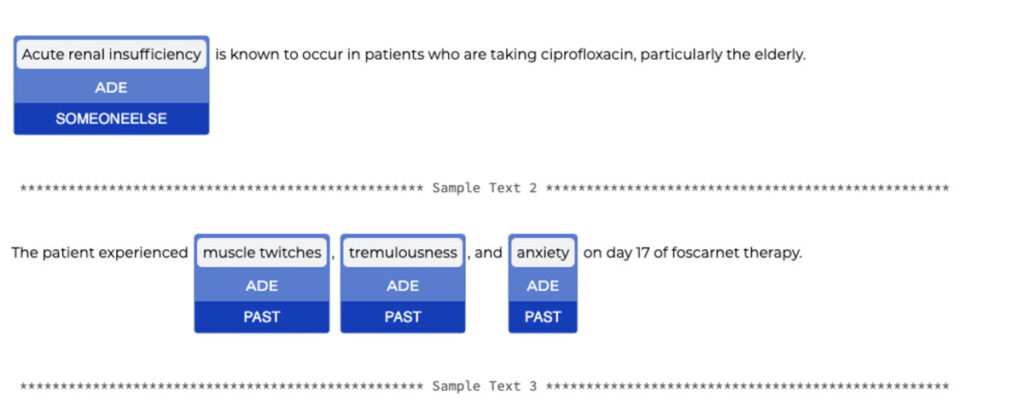
By simply adding a stage in the pipeline, we can detect the assertion status of the ADE (present, absence, occured in the past, etc).
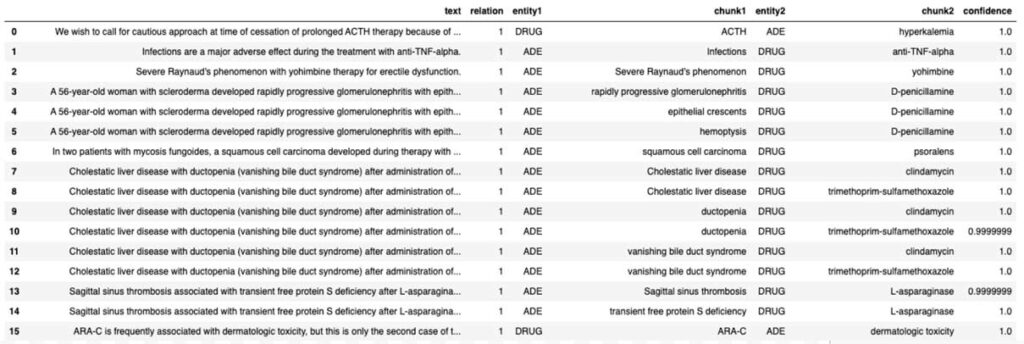
To infer the relationship status of an ADE with a clinical entity, we use a pre-trained model (re_ade_clinical), which detects the relationships between a clinical entity (in this case drug) and the inferred ADE.
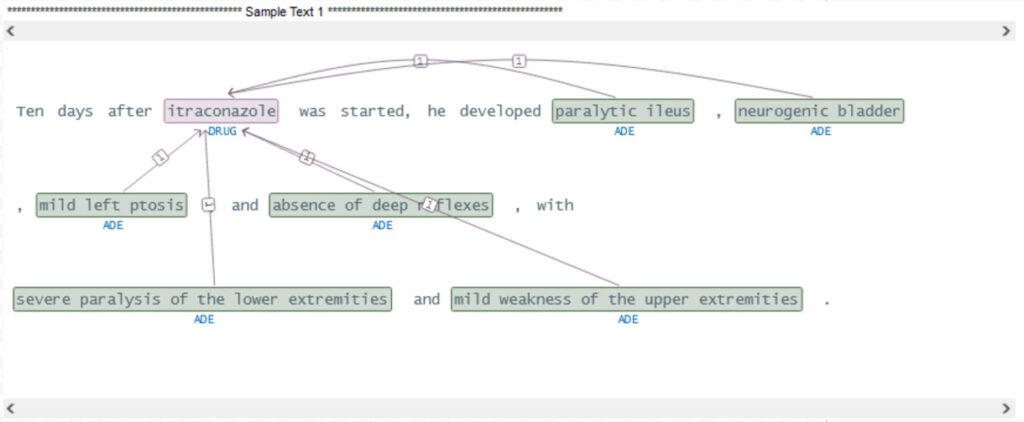
The sparknlp_display library has the ability to show relations on the raw text and their linguistic relationships and dependencies as demonstrated below.
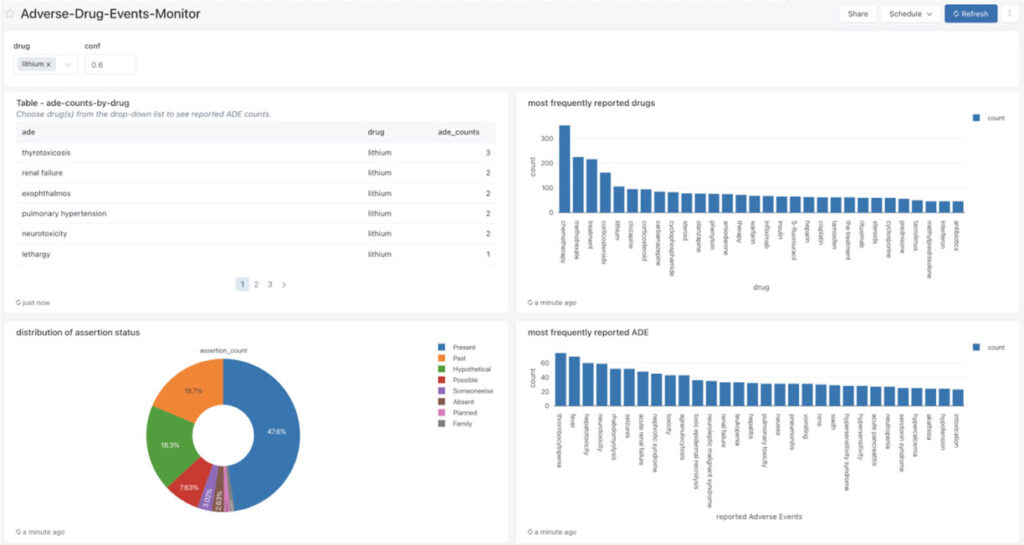
After the ADE and drug entity data has been processed and correlated, we can build powerful dashboards to monitor the frequency of ADE and drug entity pairs in real time.
Get started analyzing adverse drug events with NLP on Databricks
With this solution accelerator, Databricks and John Snow Labs make it easy to analyze large volumes of text data to help with real-time drug signal detection and safety monitoring. To use this solution accelerator, you can preview the notebooks online and import them directly into your Databricks account. The notebooks include guidance for installing the related John Snow Labs NLP libraries and license keys.
You can also visit our industry pages to learn more about our Healthcare and Life Sciences solutions.