The Challenges of Regulatory-Grade De-Identification at Scale
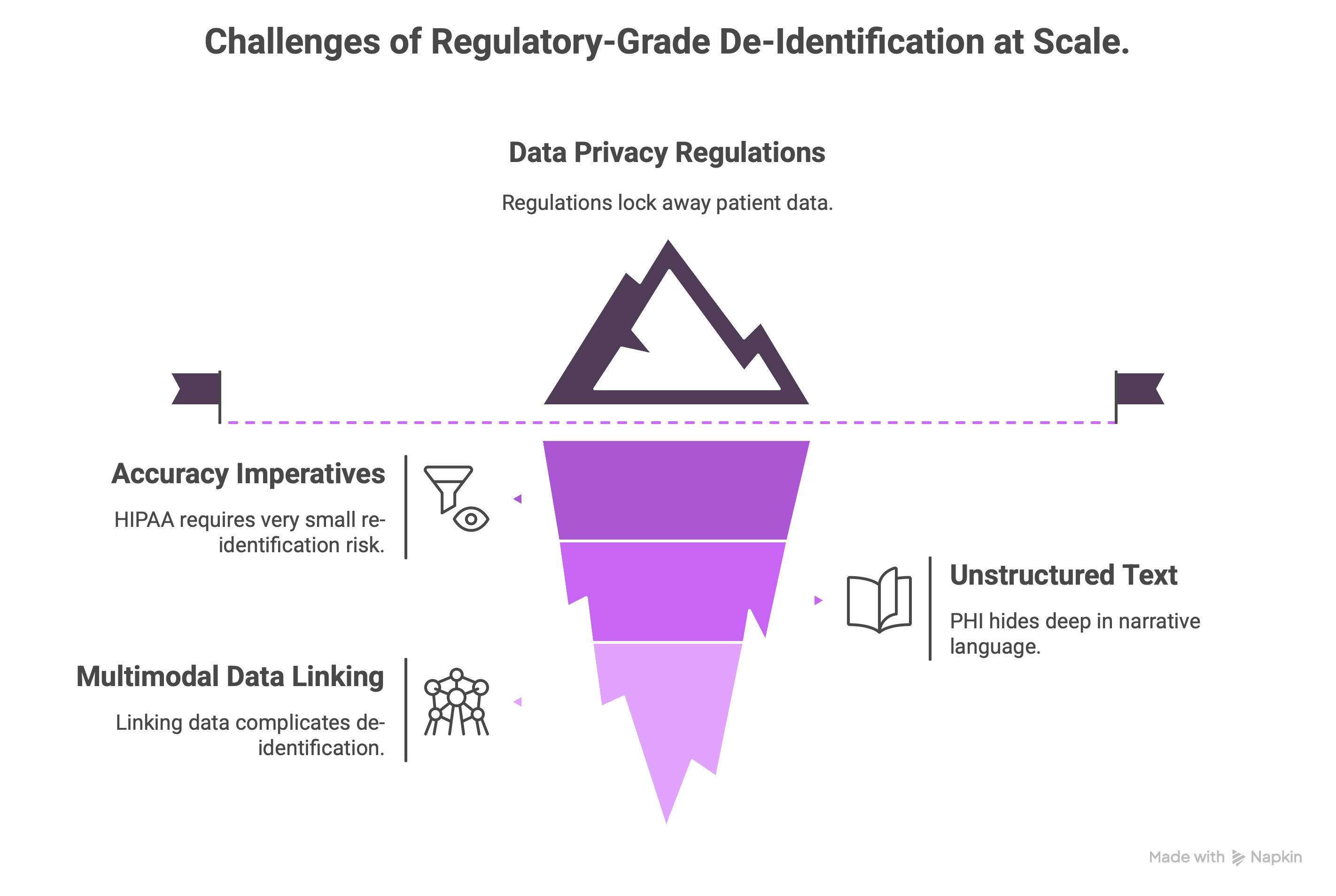
Healthcare organizations face a critical dilemma: vast volumes of patient data: free-text notes, structured fields, clinical images, even audio/video are invaluable for research, quality improvement, drug development, and analytics… yet are locked away by privacy regulations like HIPAA, GDPR, and others.
- Imperatives for accuracy: The HIPAA expert-determination method requires that the risk of re-identification is very small, with tolerance for errors below 1%. Manual review is labor-intensive, costly, and fails to scale economically.
- Unstructured text (e.g., discharge summaries, pathology reports) often hides PHI: names, dates, IDs, locations deep in narrative language. Human experts largely fail to reach the desired level of error in practice.
- Linking Multimodal data complicates matters further: images (Xrays with burned-in PHI), voice recordings, even waveform data, each need bespoke de-identification. Beyond redaction, it’s also critical to obfuscate and tokenize personal identifiers so that data about the same patient from multiple sources, modalities, and dates can still be safely linked into its de-identified form.
Why Automatic De-Identification Unlocks Major Business Value
a) Medical Data Licensing & Monetization
Regulatory-Grade de-identified clinical data sets, especially including narrative notes and images, are gold for pharma, diagnostics, and AI companies. In industries where buyers pay per dataset, manual methods are too expensive. Automatic deid enables economical, high-volume data pipelines, opening new revenue opportunities.
b) Real-World Data (RWD)
Generating RWD from EHRs, imaging archives, remote monitoring, etc., requires large-scale PHIfree data flows. Only automated deid can make this sustainable. Researchers and health systems alike benefit from enriched, comprehensive datasets with privacy protection.
c) Research Collaborations Without IRB Barriers
De-identified data under HIPAA removes the need for IRB approval and informed consent in many use cases. If you can ensure regulatory-grade de-identification without human review, collaborations with academia, startups, and other institutions become simpler, faster, and less risky.
John Snow Labs: PeerReviewed Validation of RegulatoryGrade Accuracy
Accuracy and Cost Comparison with Major Cloud & LLM Services (Text2Story@ECIR 2025)
In the 2025 Text2Story workshop at ECIR, John Snow Labs’ Healthcare NLP outperformed other leading solutions – AWS Comprehend Medical, Azure Health, and even GPT4o, at de-identifying unstructured clinical text.
- Achieved 96% F1 in PHI detection vs. 91% (Azure), 83% (AWS), and 79% (GPT4o) the only solution to reach regulatory-grade accuracy.
- Token-level and entity-level assessments confirmed very low error rates, with performance that surpasses human reviewers.
- Cost-Efficient at Scale: Operating on a fixed-cost, John Snow Labs’ locally deployment model avoids the high pertoken cloud pricing of others, making it more than 80% cheaper than Azure or GPT4o.
BillionNote, MultiLingual Deployment (ML4H 2023)
In a 2023 ML4H workshop paper, John Snow Labs demonstrated automated, humanreviewfree deidentification of 1+ billion real clinical notes, achieving over 98% coverage across seven European languages.
- It made between 50% and 575% fewer errors that the healthcare-specific services offered by AWS, Azure, and Google, and outperformed ChatGPT by ~33%arxiv.org.
- Includes PHI “surrogate” replacement to retain narrative coherence essential for downstream analytics. For example, if “John Wayne” is replaced by “Mark Twain”, it will be consistently replaced throughout the text, so that further mentions of “John” will be replaced by the obfuscated “Mark”.
- The de-identification pipeline publicly documents coverage for 30+ PHI categories: names, IDs, dates, locations, contacts, SSNs, images, complete with masking and obfuscation. This includes data fields that are not required to redact by the HIPAA Safe Harbor method but are needed in practice: clinician names, hospital names, patient profession, and certain demographic fields that must be redacted by the GDPR. Workflows
n2b2 Benchmark Record (October 2022)
Another example of John Snow Labs’ scientific rigor is the 2022 peer-reviewed paper “Accurate Clinical and Biomedical Named Entity Recognition at Scale” (Software Impacts). This study highlights how John Snow Labs delivers state-of-the-art Named Entity Recognition (NER) capabilities, integral not only for extracting medical concepts but also for enabling downstream tasks like de-identification, while remaining practical for large-scale, real-world use.
- Achieved new state-of-the-art results on 7 out of 8 leading biomedical NER benchmarks as well as on 3 major clinical concept extraction challenges including the 2014 n2c2 de-identification challenge and 2018 n2c2 medication extraction challenge medium.com+8sciencedirect.com+8academia.edu+8.
- Enterprise Grade: John Snow Labs’ solution scales natively to process hundreds of millions of records on Spark clusters, with no internet dependency (airgapped compliance) and fully customizable pipelines.
Proven Case Studies & External Validation: Real-World, Certified, and Legally Defensible De-identification
While peer-reviewed papers provide objective, benchmarked evidence of John Snow Labs’ superior de-identification accuracy, what truly sets these solutions apart is the depth and breadth of real-world adoption, validation, and certification.
John Snow Labs’ de-identification technology is not just lab-proven, it is field-proven.
Deployed across dozens of top-tier hospitals, academic medical centers, pharmaceutical companies, and data platforms, these solutions have undergone rigorous security and compliance reviews by institutional privacy officers, data protection officers, and IRB boards. More importantly, they have been subjected to independent, third-party validation under the HIPAA Expert Determination standard, a formal process where risk analysis is conducted by qualified statisticians or privacy experts to verify that the likelihood of patient re-identification is “very small.”
The result: For each deployment, these third-party assessors have issued formal certifications stating that the output is legally de-identified. This provides healthcare organizations with not just confidence, but legal protection: proof that they have met their regulatory obligations when sharing or commercializing de-identified data.
Let’s explore some of the most compelling real-world examples.
Dandelion Health: Multimodal De-identification with HIPAA Expert Determination Certification
Dandelion Health provides a platform for responsible AI development in healthcare, with access to diverse real-world data across hospitals and use cases. To enable AI research and innovation without compromising patient privacy, Dandelion needed to de-identify a wide range of data types, including structured EHR data, unstructured clinical notes, and diagnostic reports from radiology, pathology, and echocardiography.
They partnered with John Snow Labs to build a fully automated, multimodal de-identification pipeline using Spark NLP for Healthcare and domain-specific large language models. This pipeline:
- Operates at scale, handling millions of records across diverse formats and clinical specialties.
- Performs context-aware de-identification, replacing PHI with semantically appropriate surrogates to preserve the usability of the text for downstream analytics and model development.
- Was deployed on secure infrastructure, enabling full control over privacy, data flow, and logging.
To ensure compliance and legal defensibility, Dandelion brought in an independent third-party expert to review the pipeline and its output. This party conducted a formal HIPAA Expert Determination assessment and issued a certification confirming that the pipeline’s outputs were legally de-identified.
This validation enabled Dandelion to safely share data with AI researchers and commercial partners, unlocking collaboration while maintaining the highest standard of privacy protection.
Providence Health: De-identifying 700M+ Notes with Multi-Level External Validation
One of the most ambitious real-world de-identification efforts was led by Providence, one of the largest not-for-profit health systems in the U.S., operating 51 hospitals and over 800 clinics.
Providence aimed to unlock research at scale by de-identifying the entire historical archive of unstructured clinical notes across their network. This included building a pipeline that could:
- Automatically de-identify over 700 million clinical notes, with no manual review.
- Keep pace with daily updates, continuously de-identifying new incoming records.
- Maintain HIPAA compliance and avoid any re-identification risks at a scale where even a tiny error rate could result in thousands of exposed PHI instances.
To ensure that their de-identification solution was both accurate and legally defensible, Providence implemented three distinct layers of validation:
Independent Third-Party Certification
An external privacy expert conducted a full HIPAA Expert Determination process. This included reviewing the technology, performing statistical risk analysis, and analyzing real de-identified outputs.
Result: A formal certification stating that the pipeline met HIPAA’s legal de-identification requirements and that the likelihood of re-identification was very small.
Bias Testing
Recognizing that de-identification accuracy must hold across diverse populations, Providence’s internal compliance and governance teams conducted bias measurement and fairness testing. This meant evaluating the model’s accuracy across different patient demographics, such as race, gender, ethnicity, and age to ensure that PHI was correctly removed regardless of a patient’s background.
Result: The pipeline passed bias audits, with no significant disparities found across groups.
Red-Teaming by an External Security Firm
To test the robustness of the de-identification, Providence hired a global consulting firm to conduct a 90-day red-teaming engagement. The firm’s mission: attempt to re-identify any patient from a large sample of the de-identified dataset, using both internal data and external sources.
Result: After 3 months of extensive effort, not a single patient was re-identified.
Together, these three layers third-party certification, bias testing, and adversarial red-teaming provided Providence with the confidence and legal documentation to use the de-identified data for internal research, data science, and external collaborations, all without exposing themselves to privacy risk or legal liability.
Loopback Analytics: Scaling De-identification for Longitudinal Outcomes Research
Loopback Analytics is a data platform focused on helping health systems and specialty pharmacies manage and optimize outcomes for high-risk populations. Their work often involves longitudinal analysis of patient cohorts, drawing from large volumes of real-world clinical documentation.
To meet their privacy obligations and ensure scalable, efficient data sharing, Loopback deployed John Snow Labs’ automated de-identification solution for unstructured medical text. This solution processes millions of records while ensuring consistent and accurate removal of sensitive information enabling Loopback to power downstream analytics, machine learning, and real-world evidence generation without delay.
As with other customers, Loopback brought in a third-party privacy expert to evaluate and certify the de-identification pipeline. This review included:
- Assessing the model’s false negative and false positive rates on actual production samples.
- Evaluating the output for context preservation and usability in analytics workflows.
- Performing a formal HIPAA Expert Determination risk assessment.
Loopback received legal certification that their de-identified data meets HIPAA standards, allowing them to collaborate confidently with their provider clients, payers, and life science partners, all without the burden of IRB approval or complex data sharing agreements.
In all of these deployments, John Snow Labs’ technology enabled healthcare organizations to replace manual review with automation, while maintaining regulatory-grade accuracy and passing the most stringent external reviews. The result: scalable, certified de-identification pipelines that unlock the full potential of healthcare data safely, legally, and efficiently.
Conclusion: No Human Review, Regulatory-Grade De-Identification at Scale
By combining top-tier model accuracy, hybrid masking/obfuscation for context preservation, cost-effective deployment, and certification-backed results, John Snow Labs enables:
- Fully automatic de-identification across text, images, structured data, without human reviewers.
- PHI-free datasets validated under HIPAA expert-determination, ready for monetization, RWD, and research.
- Enterprise-scale pipelines capable of processing billions of notes cost-effectively.
For healthcare orgs, data aggregators, and researchers, this isn’t just technology, it’s a revolution in how protected data can be shared, analyzed, and commercialized securely, legally, and at scale.





























