Why is cancer registry automation no longer optional?
Cancer registries underpin oncology research, population health, clinical trials and value‑based care. Yet many registries remain manual, with certified tumor registrars (CTRs) reviewing pathology, radiology, clinical notes and genomics to manually abstract each case. This results in major delays, high cost and limited scalability.
As oncology becomes more data‑driven and frequently updated insights are needed for decision‑making, ranging from trial eligibility to quality reporting, the traditional manual abstraction model simply cannot keep up. Automation is therefore not optional. It is the only viable path to timely, scalable and trustworthy oncology data.
What are the challenges of current cancer registry processes?
- Volume of data: A single lab or hospital may generate thousands of pathology, radiology and clinical‑report documents monthly, most of which need review and extraction.
- Complex abstraction rules: Oncology abstraction requires detailed knowledge of staging (e.g., American Joint Committee on Cancer (AJCC) TNM rules), biomarker interpretation, multiple text sources and temporal relationships.
- Timeliness gap: According to industry sources, many registries fail to meet targets for reporting within 12 months due to resource constraints and manual bottlenecks.
- Operational cost and staffing: Certified tumor registrars are scarce, and manual abstraction limits throughput, both for facility‑based and population‑based registries.
How does automation enable real‑time oncology data?
Automation addresses each of the above gaps by combining:
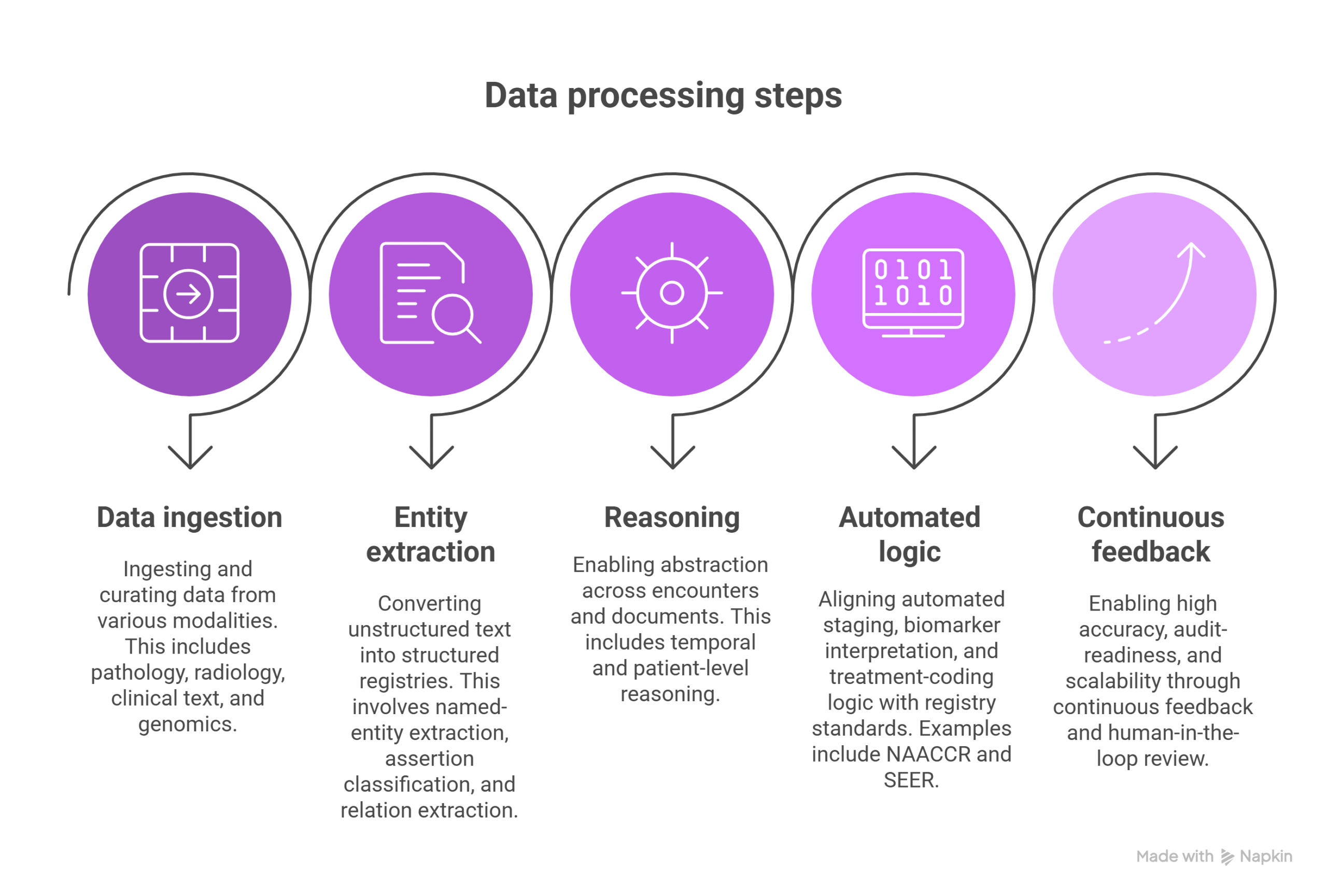
- Data ingestion and curation across modalities (pathology, radiology, clinical text, genomics)
- Named‑entity extraction, assertion classification and relation extraction to convert unstructured text into structured registries
- Temporal and patient‑level reasoning, enabling abstraction across encounters and documents
- Automated staging, biomarker interpretation and treatment‑coding logic aligned with registry standards (e.g., NAACCR, SEER)
- Continuous feedback and human‑in‑the‑loop review, enabling high accuracy, audit‑readiness and scalability
Together, these capabilities allow registry teams to shift from case‑by‑case manual abstraction to large‑scale automated processing with exception‑based human review, driving near‑real‑time availability of oncology data.
How is John Snow Labs enabling registry automation at scale?
John Snow Labs offers a comprehensive suite of tools designed for oncology registry automation:
- Under its Data Curation solution, John Snow Labs supports the creation of accurate patient registries, cohorts, quality measures and analytics from clinical documents.
- In a recent news release, John Snow Labs reported they cut cancer registry abstraction time from hours to minutes, delivering 60-100× productivity gains by using multimodal AI, agentic workflows and human oversight.
- The John Snow Labs proposition is based in a four‑part architecture: (1) data curation and extraction pipelines, (2) patient‑level reasoning, (3) oncology‑specific agents (coding logic, staging, biomarkers), and (4) registrar‑friendly UI with feedback loops.
- The platform supports multimodal processing (text, image, structured data) and audit‑ready logs, enabling registries to scale, maintain compliance and support downstream research and public‑health reporting.
What are the key use‑cases and benefits of automation?
Use‑cases
- Automated case‑finding: Filter thousands of reports to identify those that are reportable, reducing workload on registrars.
- Automated abstraction: Extract staging, histology, biomarkers, treatments and outcomes from disparate documents.
- Near‑real‑time registry updates: Enable up‑to‑date oncology data for research, trials and operations.
- Quality monitoring and analytics: Structured registry data supports analytics for outcomes, cohort identification and operational planning.
- Resource optimisation: Teams can shift focus from manual review to oversight, exception handling and higher value tasks.
Benefits
- Throughput increases: Manual abstraction takes on average 2 hours per case; automation can shorten this to minutes.
- Improved timeliness: Automated workflows reduce back‑logs in case reporting and accelerate availability of data for decision‑making.
- Cost reduction: Fewer full‑time registrars required; resources can be reallocated.
- Higher data quality: Structured extraction, standardised coding logic and continuous feedback reduce variability and errors.
- Enhanced research and analytics: Up‑to‑date registry data powers trials, real‑world evidence, population health and precision oncology.
How to implement registry automation
- Assess current workflow: Map case management from case‑finding through abstraction, coding, QA, submission.
- Establish data pipelines: Ingest pathology reports, radiology, clinical notes, genomics and structured data into unified data architecture.
- Apply extraction‑NLP models: Use entity extraction, assertion classification, relation mapping to convert text into structured fields. For example, John Snow Labs’ oncology‑specific pipelines support abstraction of tumor characteristics.
- Deploy decision‑logic agents: Implement staging, biomarker interpretation and treatment coding logic aligned with registry standards such as SEER/AJCC.
- Build registrar‑UI and feedback loop: Provide abstractors with a user interface that pre‑fills fields, shows provenance and allows corrections.
- Monitor, audit and iterate: Track accuracy metrics, error rates, throughput and adjust models continuously. Use human‑in‑the‑loop for oversight and model improvement.
- Scale and integrate: Expand from facility‑based registries to network registries, integrate into research platforms and real‑world‑evidence pipelines.
What are the challenges and how can they be mitigated?
- Data heterogeneity: Oncology data spans pathology, radiology, clinical narratives, genomics, requiring robust multimodal ingestion and processing. Automation platforms must support these modalities and maintain flexible pipelines.
- Complex abstraction rules: Staging and biomarker logic are complex and update frequently (e.g., AJCC updates every few years). The system must be configurable and maintainable.
- Regulatory compliance and auditability: Registry data is subject to standards (e.g., NAACCR, state/federal reporting), requiring full provenance, traceability, versioning and role‑based access.
- Change management: Registrars and oncology teams must trust automation. Transparent UI, exception review, and measurable accuracy are key to adoption.
- Integration into existing systems: Ensuring that automation integrates with EHRs, research systems and analytics platforms without large disruption.
Future directions: The road to real‑time oncology intelligence
- Agentic workflows: Automation agents that proactively identify new cases, coordinate abstraction, trigger alerts, and feed registries continuously.
- Multimodal abstraction: Incorporation of images (radiology, pathology slides), genomics and text into unified abstraction pipelines.
- Closed‑loop learning systems: Abstractor corrections feed back into models, improving accuracy over time and reducing human intervention further.
- Linkage to real‑world evidence and precision medicine: Registries become inputs to trial recruitment, outcome monitoring, disease progression modelling and personalized oncology.
- Networked registries and federated learning: Multiple institutions share de‑identified data, learn from each other’s models and accelerate insights across populations.
Conclusion: Why now is the time to act
With increasing pressure on oncology teams, growing data volumes and the demand for timely insights, cancer registry automation is no longer an optional efficiency project, it is fundamental to the future of oncology care, research and operations. John Snow Labs’ automated abstraction, multimodal reasoning, registrar‑friendly workflows and audit‑ready platform provide a concrete path to real‑time oncology data. Hospitals, cancer centers and registry programs that adopt automation today will be equipped for the next wave of data‑driven oncology.
FAQs
Q: Can registry automation replace certified tumor registrars (CTRs)?
A: No. Automation enables registrars to focus on exception review, oversight and high‑value tasks. Human expertise remains critical for audit, validation and interpretation of complex cases.
Q: How accurate are automated abstraction solutions compared to manual?
A: John Snow Labs reports accuracy and throughput improvements of 60‑100× when combining multimodal AI with human‑in‑the‑loop review.
Q: Does automation work for all cancer types and registries?
A: Yes, when implemented correctly. Automated workflows must support site‑specific abstraction logic, updated staging rules and multiple modalities. John Snow Labs’ approach addresses this via oncology‑specific agents.